Revolutionizing Women’s Health: A Breakthrough in Cervical Cancer Screening
Newz Daddy HealthCare Updates
In a groundbreaking development for women’s health, a pioneering medical technology startup from Ahmedabad, India, has achieved a significant milestone. IOTA Diagnostic proudly announces the approval of its revolutionary M-Strip device by the Central Drugs Standard Control Organization (CDSCO) in India. This innovative device offers a novel approach to cervical cancer screening, empowering women to conduct self-sampling in the comfort and privacy of their homes. Let’s delve into the details of this remarkable innovation and its potential impact on healthcare.
Introduction to IOTA Diagnostic and the M-Strip
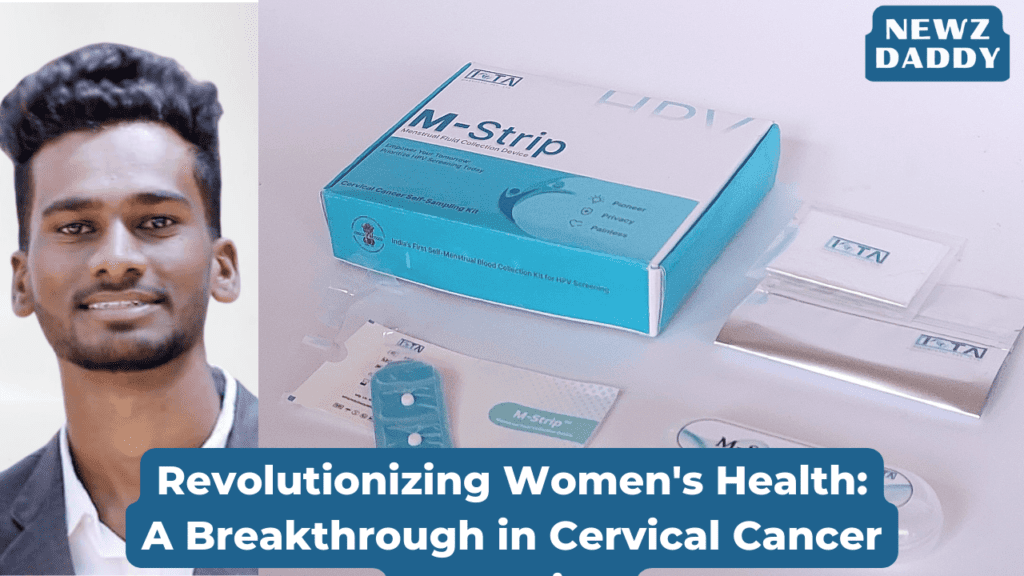
IOTA Diagnostic, founded by Mr. Vaibhav Shitole, is at the forefront of bio-sampling solutions, with a focus on enhancing women’s health. The M-Strip, developed by IOTA Diagnostic, represents a leap forward in cervical cancer screening technology. This device, conceptualized by Dr. Somesh Chandra, an esteemed oncologist from Ahmedabad, offers a convenient and non-invasive method for early detection of cervical cancer and sexually transmitted infections (STIs). Collaborating with Sterling Accuris, a leading diagnostic chain in northwest India, Dr. Chandra conducted a study that paved the way for the development of the M-Strip.
The M-Strip stands out due to its innovative design and diagnostic method. Unlike conventional screening techniques such as Pap smear microscopy and DNA tests, the M-Strip utilizes menstrual blood for sample collection. This approach capitalizes on the unique properties of menstrual fluid, allowing for the detection of Human Papillomavirus (HPV) and other infectious diseases. Moreover, the M-Strip incorporates a proprietary material known as Dried Matrix, enabling sample preservation for extended durations. This feature is particularly advantageous for patients in remote areas with limited access to healthcare facilities.
Addressing Challenges in Cervical Cancer Screening
Cervical cancer screening poses several challenges, including limited accessibility, discomfort associated with conventional methods, and societal taboos. The M-Strip addresses these challenges by offering a convenient, non-invasive, and privacy-preserving alternative. By integrating the M-Strip into sanitary pads, women can collect samples during menstruation without discomfort. This approach eliminates the need for skilled medical professionals and expensive clinical setups, making screening more accessible to a wider population.
Early detection is crucial in the fight against cervical cancer, as it allows for timely intervention and improved treatment outcomes. Unfortunately, in countries like India, where only 2% of women undergo cervical cancer screenings, many cases go undetected until they reach advanced stages. The M-Strip aims to change this narrative by encouraging routine screening through its user-friendly and cost-effective approach. By detecting precancerous lesions early on, the M-Strip has the potential to save countless lives and reduce the burden of cervical cancer on healthcare systems.
Impact on Public Health
The approval of the M-Strip by the CDSCO marks a significant milestone in the field of women’s health. With cervical cancer being the second most common cancer among women aged 15-44 years in India, the need for effective screening methods is more pressing than ever. The M-Strip not only offers a solution to this pressing healthcare challenge but also aligns with the World Health Organization’s recommendations for regular screening among women aged 25-65 years. By empowering women to take charge of their health through self-sampling, the M-Strip has the potential to revolutionize cervical cancer screening on a global scale.
The development and approval of the M-Strip would not have been possible without the collaborative efforts of various stakeholders. Mr. Vaibhav Shitole, Dr. Somesh Chandra, and Mr. Rajiv Sharma from Sterling Accuris are credited as the inventors and co-filers of the patent for the M-Strip device. Additionally, the support of organizations such as BIRAC-DBT, Sristi Innovations, Entrepreneurship Development Institute of India, i-Hub Gujarat, and STBI-Vadodara has been instrumental in facilitating the research and development process.
Future Prospects and Accessibility
Looking ahead, IOTA Diagnostic aims to enhance accessibility for women in India and underserved regions through the widespread adoption of the M-Strip. By leveraging partnerships and strategic alliances, the company plans to make the M-Strip available to a larger population, thereby contributing to the early detection and prevention of cervical cancer. Furthermore, ongoing research and development efforts will focus on expanding the utility of the M-Strip for other diagnostic applications, cementing its position as a game-changer in women’s health.
Conclusion
The approval of the M-Strip by the CDSCO represents a significant advancement in cervical cancer screening technology. By offering a convenient, non-invasive, and cost-effective solution, the M-Strip has the potential to revolutionize the way cervical cancer is detected and managed. With its emphasis on early detection and accessibility, the M-Strip is poised to make a lasting impact on women’s health worldwide. As we celebrate this milestone, let us continue to support innovative solutions that have the power to transform healthcare and save lives.